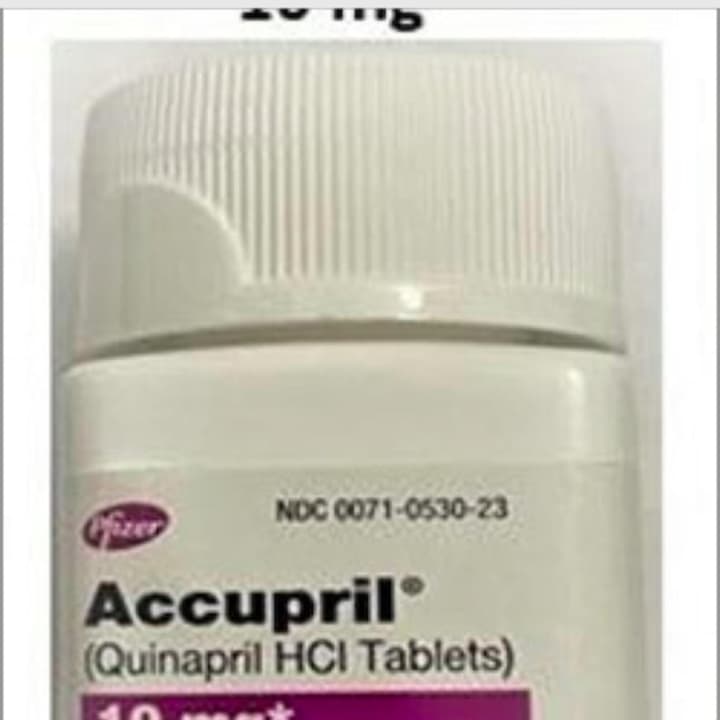
The five lots of Accupril (Quinapril HCl) tablets distributed by Pfizer to the patient level due to the presence of a nitrosamine, Nnitroso-quinapril, observed in recent testing above the Acceptable Daily Intake (ADI) level, the Food and Drug Administration announced.
"These impurities may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time," the FDA said.
Accupril has been used for the treatment of hypertension, to lower blood pressure, for more than 30 years.
"Although long-term ingestion of Nnitroso-quinapril may be associated with a potential increased cancer risk in humans, there is no immediate risk to patients taking this medication," the FDA said. "Patients currently taking the products should consult with their doctor or health care provider about alternative treatment options for them."
The product lots were distributed nationwide to wholesalers and distributors in the United States and Puerto Rico from December 2019 to April 2022:
- Accupril® (Quinapril HCl Tablets), 10 mg
- Accupril® (Quinapril HCl Tablets), 20 mg
- Accupril® (Quinapril HCl Tablets), 40 mg
Patients who are taking this product should consult with their healthcare provider or pharmacy to determine if they have the affected product, the FDA said.
Patients with the affected product should call 888-345-0481 (from 8 a.m. to 5 p.m. Monday through Friday) for instructions on how to return their product and obtain reimbursement for their cost.
Click here to read the complete FDA announcement.
Click here to follow Daily Voice Eastchester and receive free news updates.